Tag: medical devices
-
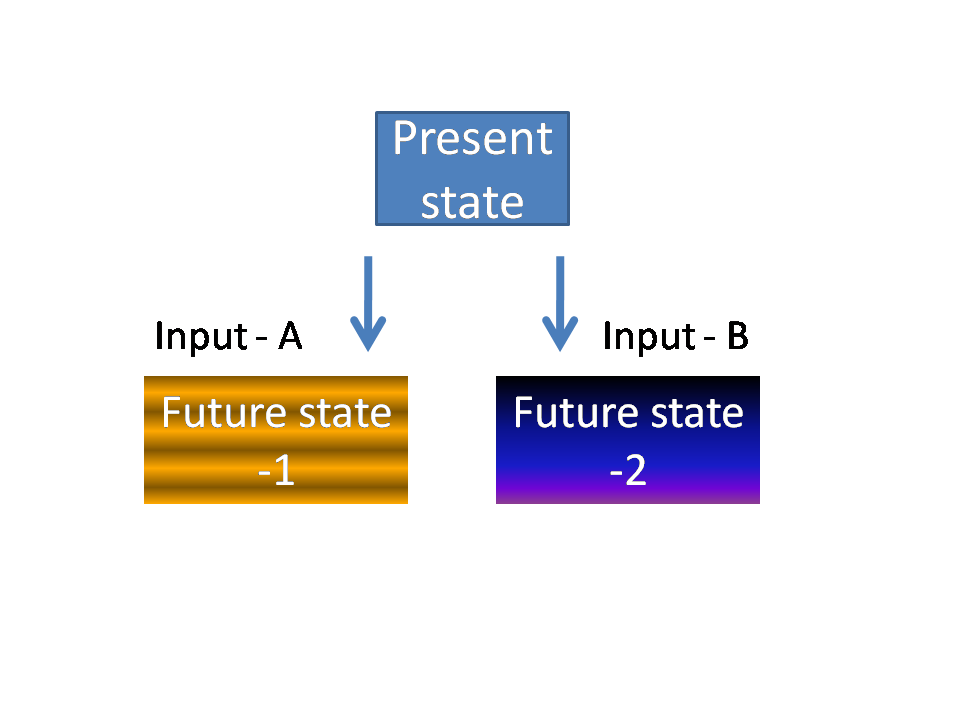
Medical software development – Finite state machines
Food and Drug Administration (FDA) regulates significant portion of medically relevant devices and drugs but it is probably not as well known that they also regulate the software that is used to power many of the studies. There are significant guidelines from FDA that are used for manufacturers of software that regulate medical devices. The…
-

Small convenient medical devices
The thinking is that sophisticated devices are needed for some biomarker studies and medicine. However, it is relatively simple to build some devices thought complicated with materials that are commonly available in electronic shops. For example, measurement of alcohol used to require sophisticated gas chromatography. With commonly available sensors, like a “MQ3 gas sensor” available…
